Molecular neurobiophysics
Where Lipid Biophysics Meets Neuronal Dysfunction
We investigate how alterations in lipid organization and membrane biophysics contribute to neurodegenerative disorders and lysosomal storage diseases, using advanced live-cell imaging and molecular biophysics approaches.
🔬 Research Projects
Lipid Droplets in Neurodegeneration
Lipid droplets (LD) are dynamic organelles involved in cellular lipid and energy metabolism. Their dysregulation has been implicated in various diseases, including neurodegenerative disorders such as Parkinson’s disease, as well as cancer and obesity. Emerging evidence suggests that changes in the neutral lipid core composition of LDs affect their biophysical properties, influencing their proteome, interactions with other organelles, and overall cellular functions.
Recently, we demonstrated that Laurdan Generalized Polarization (GP) is a sensitive tool to probe LD biophysical properties in live cells, reflecting changes in lipid composition and physiological conditions. Building on this, we aim to systematically investigate the relationship between LD composition and biophysical behavior, particularly in Gaucher disease and its link to Parkinson’s disease, and to develop robust methodologies and open-access analysis tools to support broader applications in LD cell biology research.
Project number: CZ.02.01.01/00/22_010/0008585

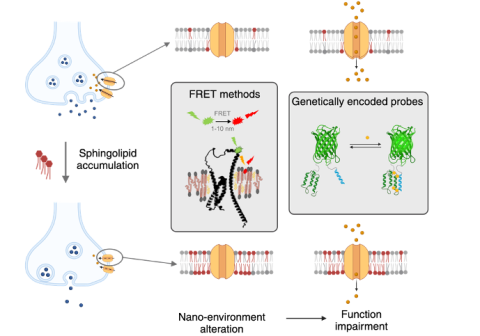
Sphingolipid-Driven Dysfunction of Transmembrane Proteins in Neuropathology
Transmembrane proteins (TMPs) are essential for neuronal function, regulating processes such as resting membrane potential, calcium homeostasis, neurotransmitter release, and synaptic vesicle fusion. Their activity depends not only on their structure but also on the precise composition of their surrounding lipid nano-environment. Disruption of this environment — particularly through sphingolipid imbalances — can impair TMP function and contribute to neuropathology.
Dysregulation of sphingolipid homeostasis has been reported in several neurodegenerative diseases, including Parkinson’s and Alzheimer’s, as well as in lysosomal storage diseases (LSDs). While in common neurodegenerative disorders the patterns of sphingolipid alterations remain inconsistent and poorly defined, sphingolipid-related LSDs offer well-characterized, genetically defined models with specific lipid changes. This makes them valuable systems for uncovering fundamental mechanisms of lipid-driven neuronal dysfunction.
By investigating these models, we aim to better understand how sphingolipid dysregulation contributes to neuropathology and to apply these insights toward deciphering the more complex lipid alterations observed in Parkinson’s and Alzheimer’s diseases. To address these processes, we will develop advanced Förster resonance energy transfer (FRET)-based methodologies and polarity-sensitive probe approaches, enabling precise characterization of the lipid nano-environment of TMPs and its functional consequences. These tools will provide new insights into how sphingolipid dysregulation contributes to neuronal pathophysiology.
👥 Team
Šárka Pokorná, PI
Šárka’s scientific interests lie in deciphering the nanoscale organization of membranes and lipid cell compartments (lipid droplets), and understanding their functional implications in neuropathology. She brings combined expertise in fluorescence method development and application, with a strong foundation in lysosomal storage diseases and their link to Parkinson’s disease.
List of publications on Google Scholar
Matúš Hodoš, PhD student
Richard Sochor, MSc student
Darina Majovska, technician
🤝 Collaborations
Liana C. Silva, iMed.ULisboa – Research Institute for Medicines, Lisbon, Portugal
Ivan Milenkovic, Medical University of Vienna, Austria
Jiří Zahradník, Charles University, Prague